- Coordination No Of Hcp
- Coordination Number Of Hcp And Fcc Lattices Respectively Are
- Coordination Number Of Hcp Youtube
- Coordination Number Of Hcp
- Coordination Number Of Hcp In 3 Dimensions
Learning Objective
- Discuss the two ways in which atoms/molecules pack in the most efficient way in crystals.
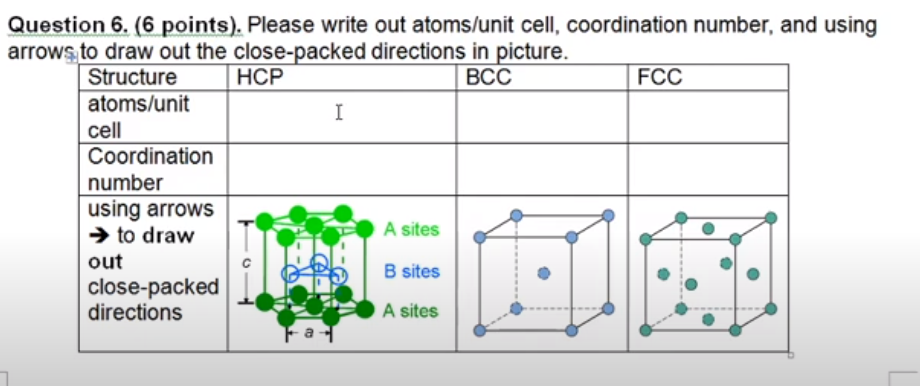
Hexagonal close packing of metal atoms is displayed interactively in 3D. Octahedral and tetrahedral holes are highlighted with ABA layer packing. Space filling and coordination number (number of nearest neighbors).Most elemental metals crystallize in the FCC (face-centered cubic), BCC (body-centered cubic, or HCP (hexagonal close packed) structures: Room temperature crystal structure Crystal structure just before it melts 2. The hexagonal closest packed (hcp) has a coordination number of 12 and contains 6 atoms per unit cell. The face-centered cubic (fcc) has a coordination number of 12 and contains 4 atoms per unit cell. The body-centered cubic (bcc) has a coordination number of 8 and contains 2 atoms per unit cell.
Key Points
- The most efficient conformation atomic spheres can take within a unit cell is known as the closest packing configuration.
- Densely packed atomic spheres exist in two modes: hexagonal closest packing (HCP) and cubic closest packing (CCP).
- The packing conformation of spheres into a unit cell can effect the physical, chemical, electrical, and mechanical properties of a given crystalline material.
Terms
- latticeA regular spacing or arrangement of geometric points.
- closest packingA phenomenon resulting in the crystal structure of atoms/molecules having their component parts as near to each other as possible.
A crystalline material’s structure is typically visualized as being composed of unit cells. These cells are periodically arranged to give rise to a crystal’s lattice structure. This section considers how the packing of atoms within unit cells contributes to a crystalline solid’s lattice structure.
Two Types of Atom Packing in a Crystal

The three dimensional structure of a solid crystalline material is established through the periodic patterning of the atoms that make up the crystal. The most efficient conformation of atomic spheres within a unit cell is known as the closest packing formation. In a three dimensional representation of this hypothetical unit cell—with the spheres packed as efficiently as possible—there are two methods to densely pack the cell.
Imagine a single layer of spheres packed into the bottom of a unit cell.
- In the first method, each successive layer of spheres covers gaps in the previous layer. Three neighboring spheres in the first layer will form a hollow space where they meet. Spheres in one layer align to fit in the hollows formed in the previous layer. The third layer aligns directly above the first layer. Because the third layer is aligned the same way as the first, this configuration is referred to as “ABA” and results in hexagonal closest packing (HCP).
- Alternatively, the gaps in the first layer are covered by the second layer. But the third layer is offset relative to the intersphere gaps of the first layer. The third layer of spheres does not align with the first layer. This configuration is referred to as “ABC” and results in cubic closest packing (CCP).
A CCP arrangement has a total of 4 spheres per unit cell and an HCP arrangement has 8 spheres per unit cell. However, both configurations have a coordination number of 12.
Coordination No Of Hcp
The packing efficiency is the fraction of volume in a crystal structure that is occupied by constituent particles, rather than empty space. In order to find this, the volume of the spheres needs to be divided by the total volume (including empty spaces) occupied by the packed spheres. For both HCP and CCP, the packing efficiency is 74.05 %.
The Importance of Packing
The arrangement of the atoms in a crystalline solid affects atomic coordination numbers, interatomic distances, and the types and strengths of bonding that occur within the solid. An understanding of atomic packing in a unit cell and crystal lattice can give insight to the physical, chemical, electrical, and mechanical properties of a given crystalline material.
Show SourcesBoundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/unit_cell
Wiktionary
CC BY-SA 3.0.

http://www.boundless.com//chemistry/definition/closest-packing
Boundless Learning
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/lattice
Wiktionary
CC BY-SA 3.0.

http://en.wiktionary.org/wiki/crystal
Wiktionary
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Crystal_structure%23Close_packing
Wikipedia
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Close-packing_of_equal_spheres
Wikipedia
CC BY-SA 3.0.

Coordination Number Of Hcp And Fcc Lattices Respectively Are
http://en.wikipedia.org/wiki/Sphere_packing
Wikipedia
CC BY-SA 3.0.
Coordination Number Of Hcp Youtube
http://www.chem1.com/acad/webtext/states/crystals-cubic.html
Steve Lower’s Website
CC BY-SA.
Coordination Number Of Hcp
http://cnx.org/content/m15058/latest/
OpenStax CNX
CC BY 3.0.
Coordination Number Of Hcp In 3 Dimensions
http://en.wikipedia.org/wiki/File:Closepacking.svg
Wikimedia Commons
CC BY-SA 3.0.
